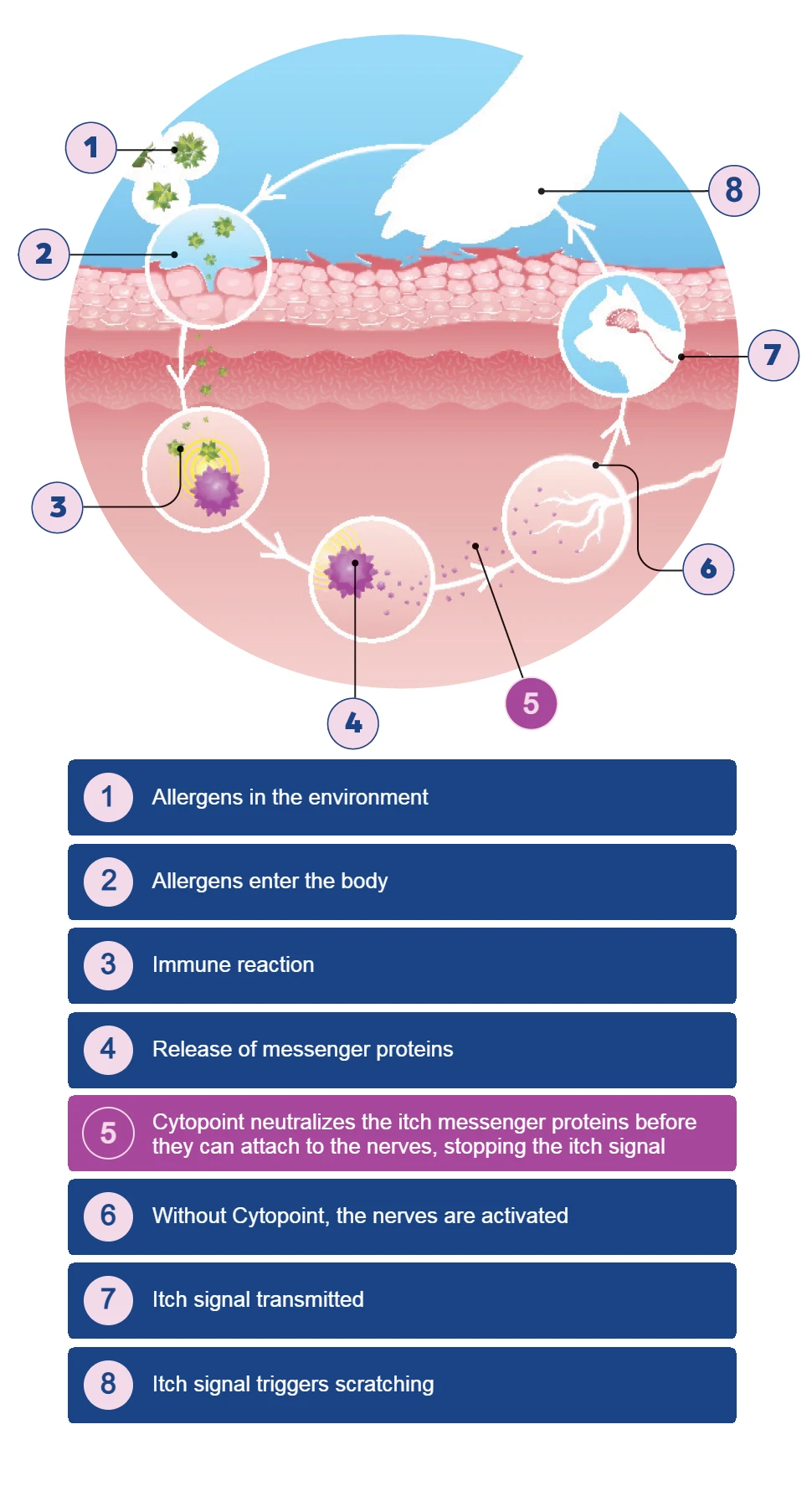
*Repeat administration every 4 to 8 weeks as needed in the individual patient.1
References
1. Michels, G. M., Ramsey, D. S., Walsh, K. F., Martinon, O. M., Mahabir, S. P., Hoevers, J. D., Walters, R. R. and Dunham, S. A. (2016), A blinded, randomized, placebo controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol. doi:10.1111/vde.123762. Gonzales AJ, Humphrey WR, Messamore JE, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24(1):48-53. doi:10.1111/j.136S-3164.2012.01098.x.
3. Olivry T, Bäumer W. Atopic itch in dogs: pharmacology and modeling. In: Cowan A, Yosipovitch G, eds. Pharmacology of Itch, Handbook of Experimental Pharmacology. 2015:357-369.
4. Michels G.M., Walsh K.F., Kryda K.A., Mahabir s"p", Walters R.R., Hoevers j"d", and Martinon O.M: ' A minimally restricted, blinded, randomized, placebo-controlled trial of the safety of lokivetmab (ZTS-00103289), a caninized anti-canine IL-31 monoclona; antibody in client-owned dogs with atopic dermatitis (2016), Abstracts, Vet Dermatol, 27:6-121. doi: 10.1111/vde.12310
5. HIII PB et al, Veterinary records 2006, 158 533-539
Zoetis® and Cytopoint are registered trademarks of Zoetis or its licensors.©2026 Zoetis Services LLC. All rights reserved.